Background: Cytogenetic abnormalities detected by fluorescence in situ hybridization (FISH) are known to differentially impact prognosis in patients with chronic lymphocytic leukemia (CLL). Deletion6q23 (del6q23) has been reported in ~2-3% of CLL patients at diagnosis; however, its prognostic significance remains indeterminate.
Methods: We identified previously untreated CLL patients from the Mayo Clinic CLL Database seen between 1/1995 - 2/2020 who had FISH performed. The clinical characteristics, concomitant cytogenetic abnormalities, time to first therapy (TTFT), and overall survival (OS) were compared between patients with del6q23 to those without this abnormality. TTFT was analyzed from FISH date until treatment or last known untreated date, accounting for competing risk of death. OS was analyzed using Kaplan-Meier methods from FISH date. In a matched (CLL-IPI risk score and sex) analysis, Cox regression analysis was used to determine the association between TTFT/OS and presence of del6q23. The Mayo Clinic IRB approved this study.
Results: A total of 3101 CLL patients were identified; their median age at diagnosis was 64 years and 66% were male. Of these patients, 42 (1.3%) had evidence of del6q23 by FISH. Compared to patients without del6q23, patients with del6q23 carried a more adverse clinical and cytogenetic profile at the time of diagnosis including advanced Rai stage (22% Rai III/IV vs. 9% Rai III/IV; P = 0.002), unmutated IGHV genes (91% vs. 43%; P < 0.001), and high/very high risk CLL-IPI (50% vs. 27%; P < 0.001).
Del6q23 was the sole abnormality in 13 patients (31%). Of the remaining patients, del6q23 was concomitantly present with del13q in 8 patients (19%), and with either del11q or del17p in 21 patients (50%). Of the 42 patients with del6q23, 29 received first line therapy: chemoimmunotherapy in 16 patients, BTK inhibitor in 7 patients, and an anti-CD20 monoclonal antibody alone in 6 patients.
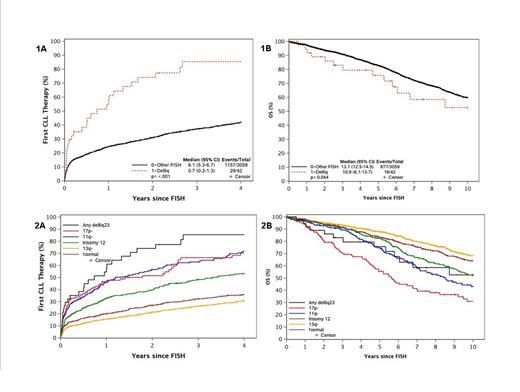
The median follow-up of the entire cohort was 7.3 years. The median TTFT in patients with del6q23 was 0.7 years while the 5-year OS was 76%. Patients with del6q23 demonstrated significantly shorter TTFT and reduced OS when compared to patients without del6q23 (Figures 1A and 1B). The shorter TTFT was also evident for patients with del6q23 compared to patients without del6q23 divided according to the Dohner risk category (Figure 2A), although there was no difference in OS between these groups of patients (Figure 2B).
Because del6q23 patients were enriched for other unfavorable prognostic markers, we identified four control patients without del6q23, matched by CLL-IPI risk score and sex, to each treatment-naïve patient with del6q23. Patients with del6q23 showed a borderline statistically significant shorter TTFT (median 0.7 years versus 3.9 years; P = 0.07) and a nonsignificant association with OS (median 10.9 years versus 10.3 years; P = 0.28).
Conclusion: Del6q23 by FISH in previously untreated CLL represents an uncommon abnormality. When compared to patients without del6q23, patients with del6q23 tend to have more adverse clinical and cytogenetic profiles at baseline, which likely confer a worse prognosis as reflected by shorter TTFT compared to patients without this abnormality.
Ding:Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; DTRM: Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees; alexion: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Astra Zeneca: Research Funding. Wang:Novartis: Research Funding; Innocare: Research Funding; Incyte: Research Funding. Braggio:DASA: Consultancy; Bayer: Other: Stock Owner; Acerta Pharma: Research Funding. Kay:Sunesis: Research Funding; Abbvie: Research Funding; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squib: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno Theraputics: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Cytomx: Membership on an entity's Board of Directors or advisory committees; Agios Pharma: Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; MEI Pharma: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees. Kenderian:Juno: Research Funding; Gilead: Research Funding; Lentigen: Research Funding; MorphoSys: Research Funding; Sunesis: Research Funding; Kite: Research Funding; Novartis: Patents & Royalties, Research Funding; Torque: Consultancy; Humanigen: Consultancy, Patents & Royalties, Research Funding; Mettaforge: Patents & Royalties; Tolero: Research Funding; BMS: Research Funding. Parikh:Ascentage Pharma: Research Funding; Merck: Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; TG Therapeutics: Research Funding; Pharmacyclics: Honoraria, Research Funding; GlaxoSmithKline: Honoraria; Janssen: Honoraria, Research Funding; MorphoSys: Research Funding; Genentech: Honoraria; Verastem Oncology: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.